
This site is intended for U.S. Healthcare Professionals only.
- U.S. Full Prescribing Information |
- Medication Guide |
- BMS Connect |
- Indication
- | Visit Patient Site
MENU


using the horizontal display
on your tablet device.

using the vertical display
on your mobile device.
Safety Profile1
Week 16 Safety Profile1
ADVERSE REACTIONS THAT OCCURRED IN ≥1% OF PATIENTS TREATED WITH SOTYKTU AND MORE FREQUENTLY THAN PLACEBO THROUGH WEEK 16 FROM PSO-1 AND PSO-21


AR=adverse reaction;
CPK=creatine phosphokinase.
*Includes upper respiratory tract infection (viral, bacterial, and unspecified), nasopharyngitis, pharyngitis (including viral, streptococcal, and unspecified), sinusitis (includes acute, viral, bacterial), rhinitis, rhinotracheitis, tracheitis, laryngitis, and tonsillitis (including bacterial, streptococcal).1
†Includes oral herpes, genital herpes, herpes simplex, and herpes virus infection.1
‡Includes mouth ulceration, aphthous ulcer, tongue ulceration, and stomatitis.1
§Includes acne, acne cystic, and dermatitis acneiform.1
- Adverse reactions that occurred in <1% of patients in the SOTYKTU group were herpes zoster1
- Infections: In the first 16 weeks, infections occurred in 29% of the SOTYKTU group (116 events per 100 PY) compared to 22% of the placebo group (83.7 events per 100 PY). The majority of infections were non serious and mild to moderate in severity and did not lead to discontinuation of SOTYKTU. The incidence of serious infections were reported in 5 patients (2.0 per 100 PY) treated with SOTYKTU, and 2 patients (1.6 per 100 PY) treated with placebo
PY=patient year.
Week 16 Safety Profile (≥5%)2
AEs OCCURRING IN ≥5% OF PATIENTS IN ANY ACTIVE TREATMENT GROUP WEEKS 0-16 FROM POOLED CLINICAL TRIALS (PSO-1 AND PSO-2)2

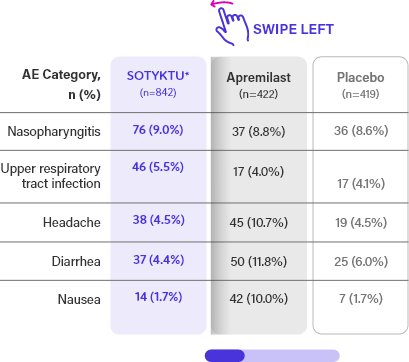


AE=adverse event.
*Includes 2 patients in SOTYKTU arm that were excluded from primary and secondary endpoint analyses.1
- Studies were not designed to compare the safety of apremilast to SOTYKTU. Some of the observed safety rates for apremilast may differ from those previously reported. Please refer to the apremilast Full Prescribing Information
SAFETY PROFILE THROUGH
WEEK 521,2
Adverse events for SOTYKTU, apremilast, and placebo2
PSO-1 AND PSO-2 POOLED SAFETY, WEEKS 0-522*


*Includes AEs between first dose and 30 days following last dose or rollover to long-term extension study.2
†Includes 2 patients in SOTYKTU arm that were excluded from primary and secondary endpoint analyses.1
AE=adverse event; AR=adverse reaction; EAIR=exposure-adjusted incidence rate; PY=patient years.
- Studies were not designed to compare the safety of apremilast to SOTYKTU. Some of the observed safety rates for apremilast may differ from those previously reported. Please refer to the apremilast Full Prescribing Information
- 1,681 patients were randomized to receive SOTYKTU 6 mg (840 patients), placebo (419 patients), or apremilast 30 mg twice daily (422 patients). All patients randomized to placebo switched to SOTYKTU at Week 16. All other patients remained in their original treatment group until Week 24, at which point patients could have continued on the same treatment or been switched to SOTYKTU or placebo1
- The most common serious infections reported were pneumonia and COVID-191
- Malignancies (excluding non-melanoma skin cancer) through Week 52 (total exposure of 986 PY with SOTYKTU) were reported in 3 patients treated with SOTYKTU (0.3/100 PY)1
- During clinical trials, including an open-label extension trial, 3 SOTYKTU patients (0.1/100 PY) developed lymphoma1
- Two deaths occurred in the SOTYKTU group while one death occurred in each apremilast and placebo group2
- One death occurred during Weeks 0-16 in a patient who discontinued SOTYKTU after 4 days of treatment due to prohibited medication (leflunomide) and died 9 days later due to sepsis and heart failure. One additional death occurred during Weeks 16-52 due to hepatocellular carcinoma in a patient with a history of HCV infection and liver cirrhosis
- The EAIR of adverse reactions in patients treated with SOTYKTU from Week 0 through Week 52 without switching treatment did not increase compared to the rate observed during the first 16 weeks of treatment1
HCV=hepatitis C virus.
Established safety profile through 3 years*
OVERALL SAFETY SUMMARY
(AS-TREATED POPULATION)†




- Enrollment for LTE trial began in August 20196
*Patients had varying lengths of treatment exposure.4
†Analysis includes 2 patients in the SOTYKTU arm who were excluded from primary and secondary endpoint analyses.
‡The LTE safety analysis represents the pooled POETYK PSO-1 and PSO-2 populations and patients enrolled in the LTE who were blindly switched from SOTYKTU, apremilast, or placebo to open-label SOTYKTU. All patients in the parent trials were eligible to enter the LTE after 52 weeks, regardless of initial treatment.3,4
§Data cutoff date of October 1, 2021. 79.0% of patients had total SOTYKTU exposure for ≥1 year and 39.9% for ≥2 years.3
||Data cutoff date of June 15, 2022. 77.6% of patients had total SOTYKTU exposure for >12 months and 22.4% for >36 months.4
AE=adverse event; EAR=exposure-adjusted incidence rate; LTE=long-term extension; PY=patient-years; QD=once daily; SAE=serious adverse event.
- There were 2 deaths in the SOTYKTU arm through 1 year. An additional 8 deaths were reported in the LTE up to the cutoff date3,4
- Six of these deaths were attributed to
COVID-19, 1 was attributed to a ruptured thoracic aortic aneurysm (not deemed to be drug-related), and 1 was attributed to an unknown cause
Peer Perspectives: Safety (Week 16 and 52)













 See efficacy
See efficacy