PROVEN SUPERIOR CLEARANCE VS APREMILAST IN H2H STUDIES
Key Secondary Endpoints

SEE THE SUPERIOR KEY SECONDARY ENDPOINTS FOR SOTYKTU VS APREMILAST IN STUDIES
IN POETYK PSO-1
~7 out of 10 psoriasis patients achieved PASI 75 with SOTYKTU1,2
PASI 75 RESPONSE RATES, WEEKS 16 AND 24 (NRI)1,2
Data shown is from PSO-1 (NRI)
- PASI 75 vs apremilast at Week 16 and Week 24 were key secondary endpoints1
| * | P<0.0001 vs apremilast.2 |
BID=twice daily; H2H=head-to-head; NRI=non-responder imputation; PASI=Psoriasis Area and Severity Index; PASI 75=≥75% improvement from baseline in PASI score; QD=once daily; sPGA=static Physician’s Global Assessment. sPGA response defined as sPGA score of 0 or 1 with ≥2-point improvement from baseline.
Demonstrated superior response rates vs apremilast1,3
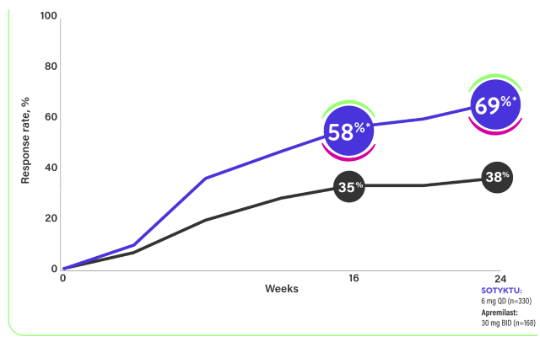
PASI 75 response rates in POETYK PSO-2 (key secondary endpoints)1,3
- In POETYK PSO-2, PASI 75 response rates at Week 16 were 53% for SOTYKTU (n=511) vs 40% for apremilast (n=254); P=0.0004. At Week 24, PASI 75 response rates were 58% for SOTYKTU (n=511) vs 38% for apremilast (n=254): P<0.0001
Maintenance of response for those on SOTYKTU
In PSO-1, PASI 75 responders at Week 24 who maintained response at Week 52 (additional endpoint)1

of patients on SOTYKTU who achieved PASI 75 at Week 24 maintained it at 1 year
(n=187/228)
Maintenance of response in POETYK PSO-2 (additional endpoints)1
- To evaluate maintenance and durability of response, patients in PSO-2 who were originally randomized to SOTYKTU and were PASI 75 responders at Week 24 were re-randomized to either continue treatment on SOTYKTU or be withdrawn from therapy (ie, receive placebo)
- At 1 year, 80% (n=119/148) of patients who continued on SOTYKTU maintained PASI 75 compared to 31% (n=47/150) of patients who were withdrawn from treatment with SOTYKTU
PASI 75 Response Rate at Week 52 (additional endpoint)2
- In PSO-1, 65% of patients receiving continuous SOTYKTU (n=330) achieved PASI 75 at Week 52
- PASI 75 at Week 52 was not a ranked primary or key secondary endpoint and was analyzed descriptively. In POETYK PSO-2, the same analysis at Week 52 for patients receiving continuous SOTYKTU is not available due to the trial design and forced re-randomization
ARE YOU READY TO EXPLORE MORE? SELECT A TOPIC BELOW.
- SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Data on file. BMS-REF-DEU-0021. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
IN POETYK PSO-1
SOTYKTU patients saw ~2x the PASI 90 response rate vs apremilast1,2
PASI 90 RESPONSE RATES, WEEKS 16 AND 24 (NRI)1,2

Data shown is from PSO-1 (NRI)
- PASI 90 at Week 16 and Week 24 were key secondary endpoints1
| * | P=0.0002 vs apremilast.2 |
| † | P<0.0001 vs apremilast.2 |
BID=twice daily; NRI=non-responder imputation; PASI=Psoriasis Area and Severity Index; PASI 90=≥90% improvement from baseline in PASI score; QD=once daily.
SOTYKTU showed superior PASI 90 response rates vs apremilast1,3
PASI 90 response rates vs apremilast in POETYK PSO-2 (key secondary endpoints)1,3
- In POETYK PSO-2, PASI 90 response rates at Week 16 were 27% for SOTYKTU (n=511) and 18% for apremilast (n=254); P=0.0046. At Week 24, PASI 90 response rates were 32% for SOTYKTU (n=511) and 20% for apremilast (n=254); P=0.0002
Maintenance of response for those on SOTYKTU
PASI 90 responders at Week 24 who maintained response at Week 52 (additional endpoint)1

of patients on SOTYKTU who achieved PASI 90 at Week 24 maintained it at 1 year
(n=103/140)
PASI 90 Response Rate at Week 52 (additional endpoint)2
- In PSO-1, 44% of patients receiving continuous SOTYKTU (n=330) achieved PASI 90 at Week 52
- PASI 90 at Week 52 was not a ranked primary or key secondary endpoint and was analyzed descriptively. In POETYK PSO-2, the same analysis at Week 52 for continuous SOTYKTU use is not available due to the trial design and forced re-randomization
ARE YOU READY TO EXPLORE MORE? SELECT A TOPIC BELOW.
- SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Data on file. BMS-REF-DEU-0021. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
IN POETYK PSO-1
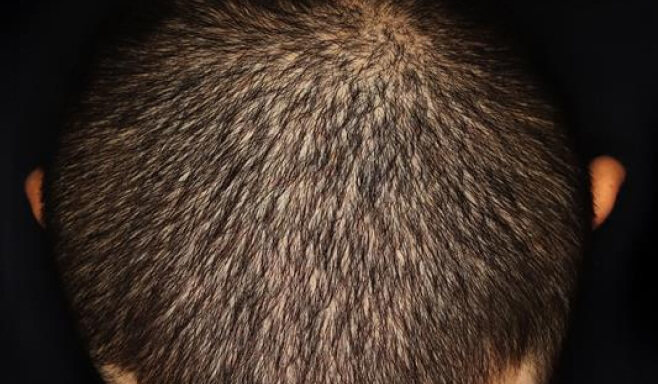
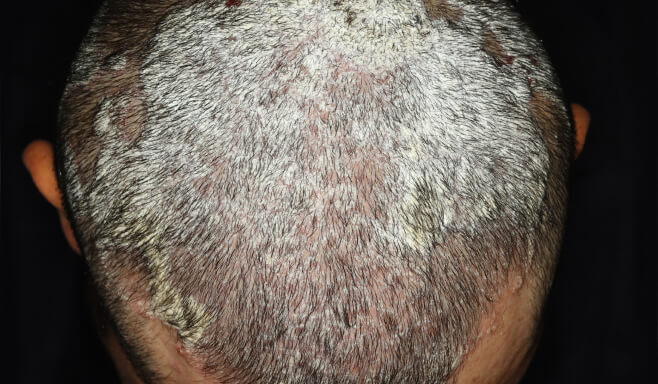
7 out of 10 SOTYKTU patients achieved a clear or almost clear scalp1,2
SS-PGA 0/1 RESPONSE RATES AT WEEK 16 (NRI)1,2*

- ss-PGA 0/1 at Week 16 was a key secondary endpoint1
- In POETYK PSO-1, ss-PGA 0/1 at Week 16 was 17% for placebo (n=121)1,2
ss-PGA 0/1 response rates in PSO-21,3*
- In PSO-2, ss-PGA 0/1 response at Week 16 was 60% for SOTYKTU (n=305), 37% for apremilast (n=166), and 17% for placebo (n=173); P<0.0001 for both
Scalp response rates at Week 24 (additional endpoint)2,3*
- In PSO-1, ss-PGA 0/1 was 72% at Week 24 for SOTYKTU (n=209) and 43% for apremilast (n=110)
- In PSO-2, ss-PGA 0/1 was 59% at Week 24 for SOTYKTU (n=305) and 42% for apremilast (n=166)
ss-PGA 0/1 at Week 24 was not a ranked primary or key secondary endpoint and was analyzed descriptively.
| * | Included only patients with baseline ss-PGA score of ≥3.1 |
| † | P<0.0001 vs apremilast.2 |
| ‡ | P<0.0001 vs placebo.2 |
BID=twice daily; NRI=non-responder imputation; QD=once daily; ss-PGA 0/1=scalp-specific Physician’s Global Assessment, patients achieving clear (0) or almost clear (1) skin.
Photos of a patient with moderate-to-severe plaque psoriasis treated with SOTYKTU in a non-registrational clinical trial (NCT05478499).4
Individual results may vary.
ARE YOU READY TO EXPLORE MORE? SELECT A TOPIC BELOW.
- SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51. doi:10.1016/j.jaad.2022.08.061
- Data on file. BMS-REF-DEU-0148. Princeton, NJ: Bristol-Myers Squibb Company; 2024.